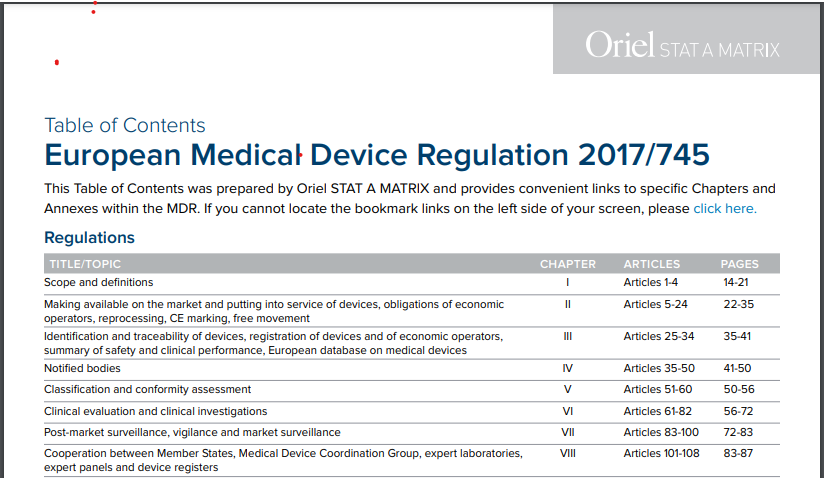
If you have downloaded the 175-page European Medical Device Regulation PDF but are frustrated that it does not contain a Table of Contents, Oriel STAT A MATRIX has come to your rescue. We have taken the official MDR regulation as published on May 5, 2017 and added a very easy-to-navigate clickable MDR Table of Contents all in one single PDF.
We’ve added internal links so you can quickly access every Chapter, Article, and Annex!
Inside the Table of Contents for MDR 2017/745, you’ll find quick links to every significant section, including:
If you need help with MDR transition strategy, gap assessments, or simply getting up to speed quickly on the MDR requirements, check out our MDR training and consulting options. Oriel STAT A MATRIX has been assisting medical device companies with QA/RA compliance for decades and we can help you smoothly transition to the MDR.

US OfficeWashington DC
EU OfficeCork, Ireland



UNITED STATES
1055 Thomas Jefferson St. NW
Suite 304
Washington, DC 20007
Phone: 1.800.472.6477
EUROPE
4 Emmet House, Barrack Square
Ballincollig
Cork, Ireland
Phone: +353 21 212 8530