Its 3:00 p.m. on Friday and you’re ready for the weekend after a busy week. All of a sudden you are jolted back to reality by an email about a serious incident at a French hospital involving your company’s heart monitor. Details are sketchy and somewhat lost in translation. It’s now 9:00 p.m. at your office in Europe. They are closed for the weekend and you can’t get more details. Can it wait until Monday’
If you’ve been in your role for some time, you already know that incidents such as this do occur and you have no control over the timing. In the case of very serious incidents, your response time is limited, sometimes as little as 48 hours. In some markets such as Australia, Brazil, and Europe, regulators want to know about very serious incidents even before you have had time to investigate them. Because you may not have much time to look into an incident let alone research the reporting requirements you need to be aware of what is reportable in each major market and when.
Every country has different rules about what needs to be reported and how quickly. But, in general, you need to file a report with a medical device Regulatory Body when:
Generally, if your labeling is sufficient, reporting is not required when:
The moment you become aware of an incident you believe to be reportable, the clock starts. It is vital that you quickly ascertain how much time you have to report the incident, which will depend on its severity. If a death or serious injury has occurred you have between 2 and 10 days to report, as shown in the table below. Less serious events/incidents can be reported in 15 to 30 days. Keep in mind that these requirements are calendar days, not work days. For example, if you learn of a death involving your device on Friday at 11:00 a.m., you’ll need to submit a preliminary incident report by Sunday if it happened in Australia. You can amend your preliminary report with additional information later. Also, if you become aware of an incident but are unsure if it is reportable, submit a report anyway. Its important to remember that filing a report on an incident/event is not an admission of either liability or that your device caused or contributed to the event. When in doubt, report.
When incidents happen, you need to be prepared to investigate them quickly. That’s important because you obviously want to ensure that no further harm is done to patients or users, but also because Regulatory Authorities mandate a speedy response. Shown below are the basics of events that may be reportable and how soon they need to be reported. Keep in mind that this is not a complete list and every country has exceptions and details that could change your reporting responsibilities.
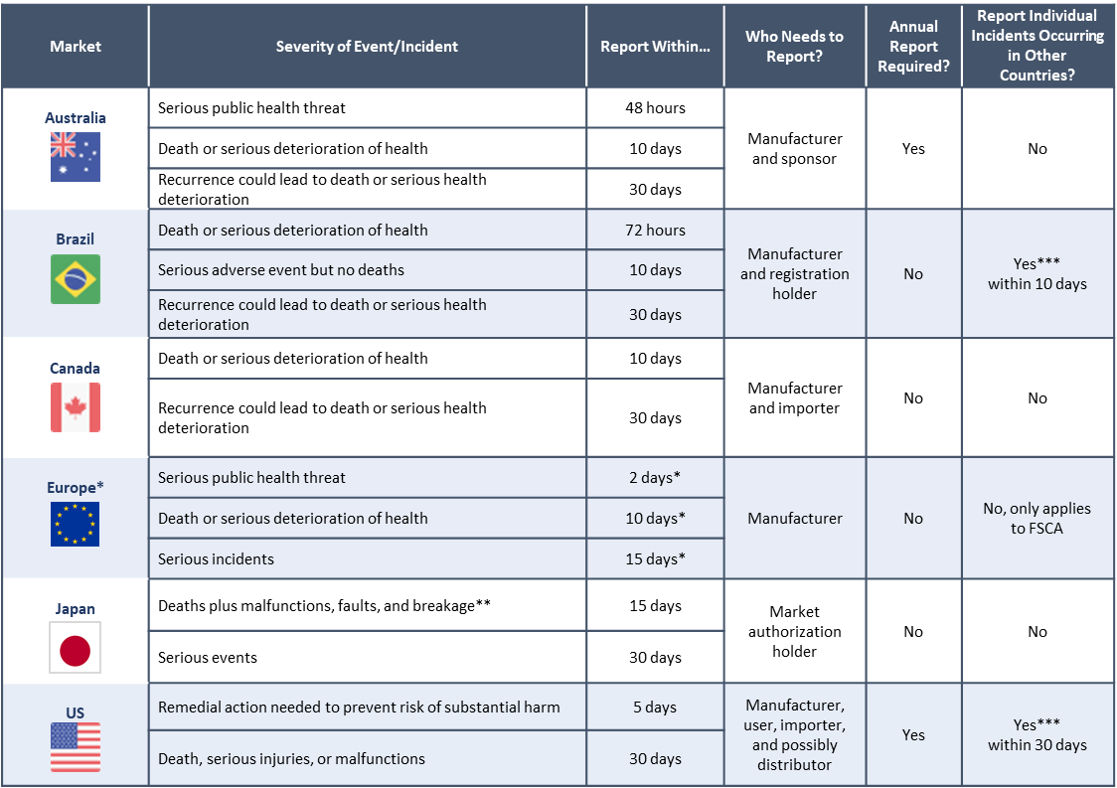
* European requirements are for the Medical Device Regulation (2017/745).
** Japan has specific reporting timelines for incidents involving device malfunctions, breakages, and fault that could lead to serious events.
*** Only required if you also sell the same device in this market. Types of reportable events vary by country. The US FDA requires the reporting of all ex-US events but in Brazil, for example, this only applies to deaths, serious deterioration of health, and counterfeit devices.
As the saying goes, The devil is in the details. You don’t want to miss a deadline because you thought something was not reportable or because you thought it was less serious than a Regulatory Authority perceived it to be. Its far better to be on the safe side and report too early than too late. Also, it’s easy to forget that a reportable incident occurring in Europe, for example, might also trigger reporting requirements in Brazil or the US if you also sell the device there. Never assume that a regulator at ANVISA would not know about your incident in France. Also, once the Eudamed database goes live in Europe in early 2020, incident data will be publicly viewable, much as it is today through the US FDAs MAUDE database.
We hope you enjoyed this quick high-level overview on incident reporting timelines. If you would like to learn a lot more about incident reporting and complaint handling, check out our series of four blog posts on the topic. Also, you can delve even deeper into this topic in our intensive training class on complaint handling ad vigilance reporting, which is held in various cities around the US.

US OfficeWashington DC
EU OfficeCork, Ireland



UNITED STATES
1055 Thomas Jefferson St. NW
Suite 304
Washington, DC 20007
Phone: 1.800.472.6477
EUROPE
4 Emmet House, Barrack Square
Ballincollig
Cork, Ireland
Phone: +353 21 212 8530