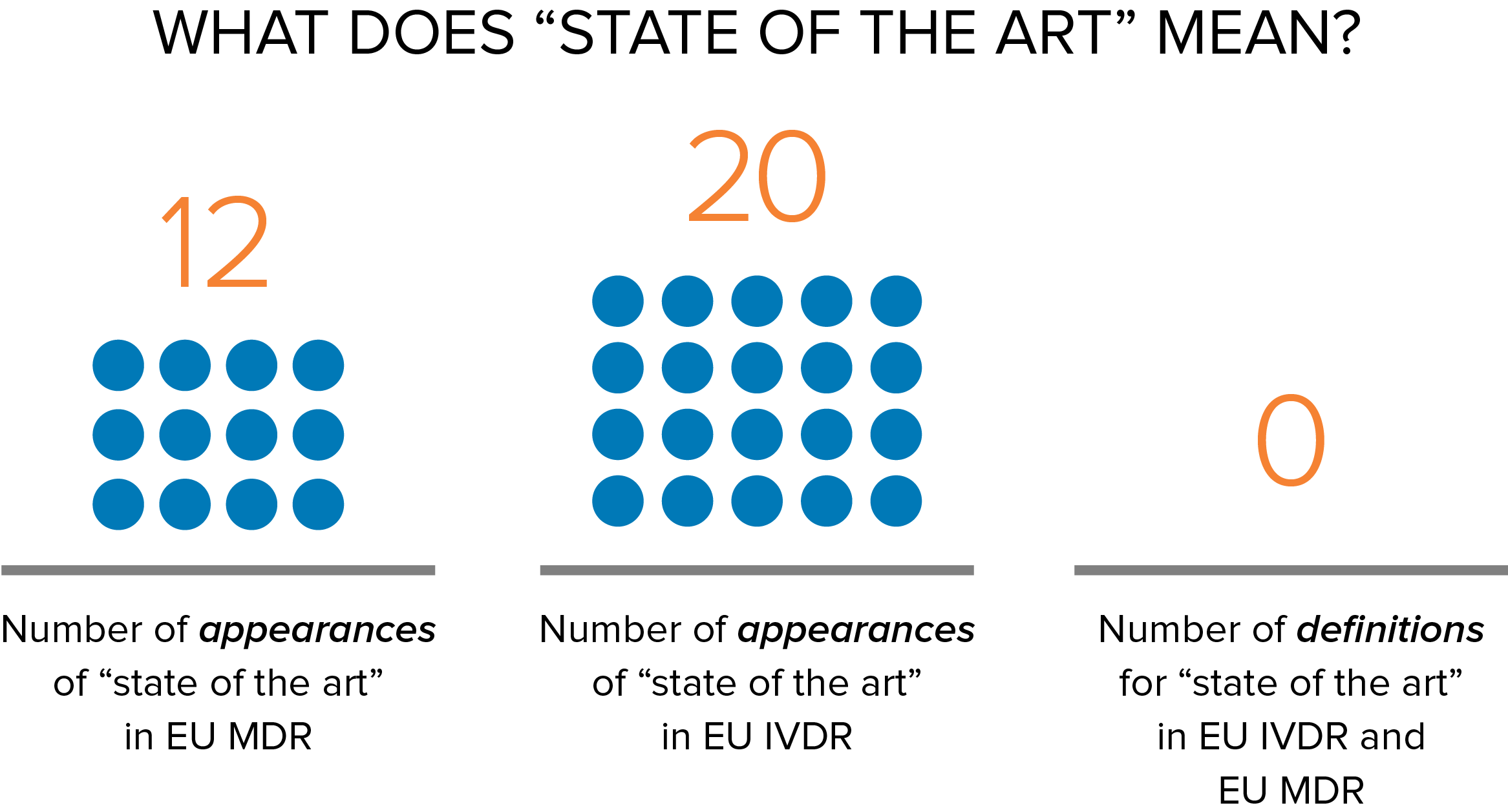
Confused? It’s understandable. Most of us think of state of the art as describing the latest and most advanced stage of a technology. For example, most people would consider a bionic eye to be state of the art. Yet, this notion of state of the art isn’t what is meant in the context of the EU MDR, IVDR, and risk management. As such, manufacturers often face challenges in assessing state of the art in design and postmarket surveillance. Some of the confusion arises because, in the medical device and IVD regulatory arena, state of the art refers only to products that are developed and approved for sale in the marketplace, not cutting-edge technology in development. As we all know, there is a very big difference between a new state-of-the-art digital imaging system that is undergoing trials and one that already has CE Marking. For EU medical device regulators, the latter is considered state of the art, but the former is not until it has CE Marking.
Here’s one way to think about this. Ask yourself: What is state of the art in cars these days? You might think of self-driving cars. But the reality is that while self-driving cars are highly innovative, they don’t represent the widespread commercially available stage of technology. In the context of the EU Medical Device Regulation, state of the art is really the middle of a bell curve in innovation. A Model T certainly isn’t state of the art, but cars with side curtain airbags, Bluetooth and backup cameras could be considered state of the art under the ISO 14971:2019 3.28.
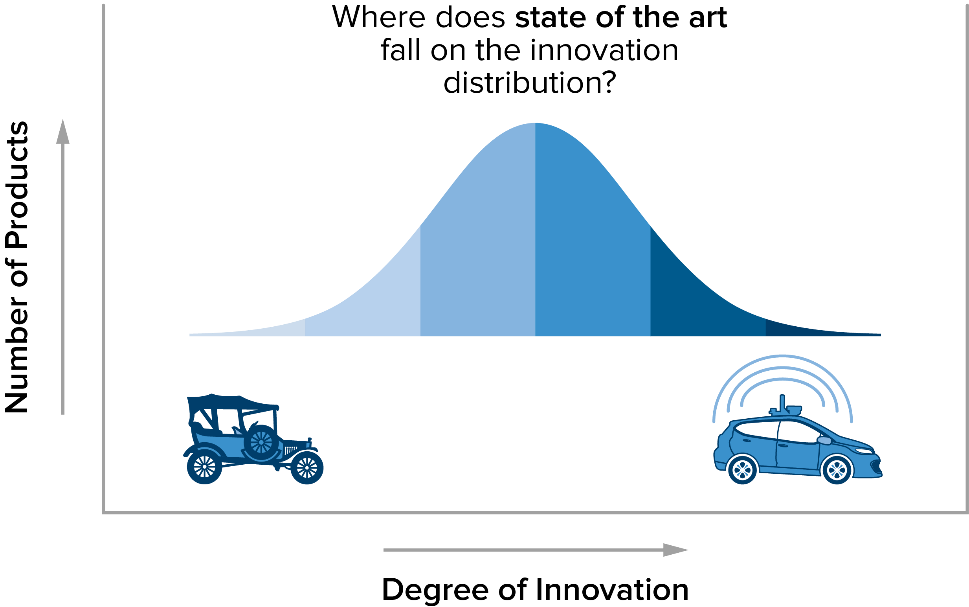
A note associated with this definition from ISO 14971:2019 3.28 adds more insight: “The state of the art embodies what is currently and generally accepted as good practice. The state of the art does not necessarily imply the most technologically advanced solution.” Thus, it is more useful to think of state of the art as meaning “the current state of all competitive treatment options.”
If your medical device or IVD is not the most technologically sophisticated product on the market but has a sparkling safety record, possesses solid clinical data, and follows all the latest standards, does that mean it still meets best practices and qualifies as state of the art in the eyes of EU regulators?
Maybe.
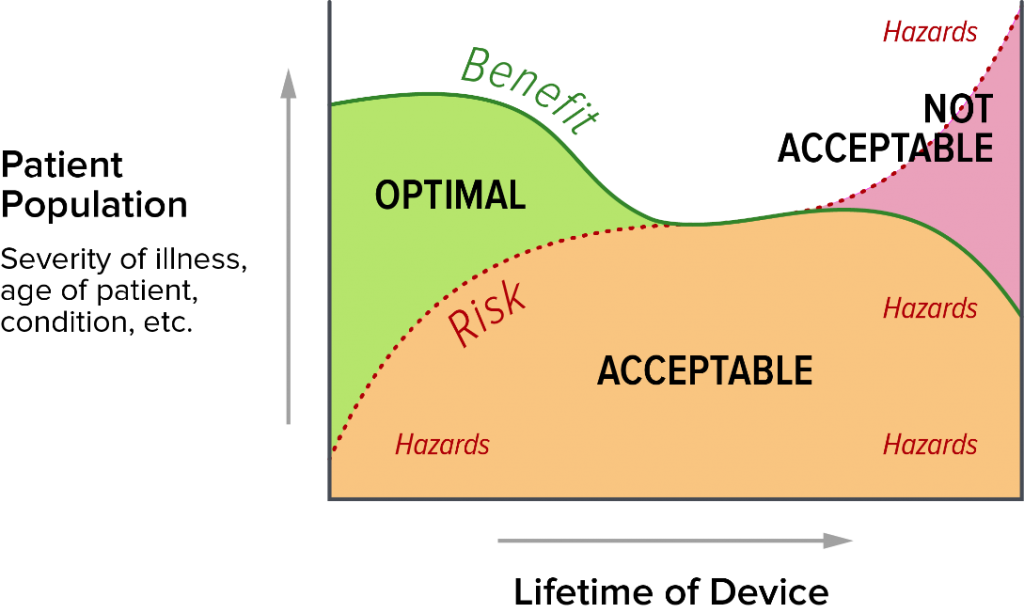
It also depends on the benefit-risk balance over the life of the device. As the device ages, assuming no new versions are introduced, it becomes less state of the art (like a Model T). It also has less benefit as newer treatment options are developed. Even if the risk profile is unchanged for your device, there will be less risky devices on the market.
In the EU MDR and associated MEDDEV 2.7/1 rev 4 guidance, you are expected to demonstrate that you have conducted a thorough analysis of the current state of the art. Notified Bodies are increasingly looking to see that you have performed an in-depth assessment of alternative treatment methods for the same indications thus, you can expect more scrutiny during your next surveillance audit.
You are required to take state of the art into account when assessing the risk acceptability of your devices, adopting risk control measures, and assessing the clinical benefit of your devices. As such, state of the art is critical in assessing the benefit-risk ratio of the device, and you need to take it seriously in your clinical evaluation report (CER). If your device has been on the market for decades and there are two devices that are technically superior and present lower risk than yours, they reduce the benefit of your device and increase the risk side of your benefit-risk equation.
MEDDEV 2.7/1 rev 4 clarifies the definition and objectives for establishing state of the art, and also provides guidance on content and methods. MEDDEV 2.7/1 rev 4 describes state of the art as current knowledge in the corresponding medical field, such as applicable standards and guidance documents, information related to the medical condition managed with the device, and its natural course, benchmark devices, or other devices and medical alternatives available to the target population. Description of state of the art provides a framework for manufacturers and Notified Bodies to assess the devices safety and performance.
In the new IVDR, it is required that manufacturers specify and justify the level of clinical evidence necessary to demonstrate conformance in view of the device characteristics and intended purpose. Clinical evidence, performance evaluation, and study requirements for IVDs are defined related to the state of the art in medicine. Clinical evidence should demonstrate, with reference to state of the art, that clinical benefit will be achieved and that devices are safe.
One of our most popular training courses focuses on medical device risk management. Recently updated for ISO 14971:2019, this course takes a deep dive into all things risk and covers risk-related issues in the MDR, MDSAP, CERs, and (of course) ISO 14971. We also offer a very popular EU MDR training course and one focused on the IVDR. Our consulting is also available to help with all things MDR.

US OfficeWashington DC
EU OfficeCork, Ireland



UNITED STATES
1055 Thomas Jefferson St. NW
Suite 304
Washington, DC 20007
Phone: 1.800.472.6477
EUROPE
4 Emmet House, Barrack Square
Ballincollig
Cork, Ireland
Phone: +353 21 212 8530