Understanding the EU IVDR Compliance Deadlines
The EU In Vitro Diagnostic Regulation (IVDR) (EU 2017/746), which went into effect on May 26, 2022, replaces the In Vitro Diagnostic Directive 98/79/EC (IVDD). It introduces stricter requirements, including new risk classifications, increased clinical evidence requirements, and post-market surveillance. Compliance with IVDR is being phased in, with specific deadlines that manufacturers must meet in order to maintain market access.
With the IVDR, the European Commission introduced a pragmatic approach to implementing it, announcing new rules and giving more time for manufacturers of legacy devices (those with existing certification under the IVDD) to transition to the new requirements without compromising safety and mitigating the risk of product shortages.
The initial transition periods were staggered, with high-risk IVDs having a shorter period than lower-risk IVDs. Article 110 of the IVDR provided a bridge for IVD devices that were legally sold under the IVDD before May 26, 2022 and devices that were sold after May 26, 2022 but had a valid certificate as stated in Article 110(2), which could continue to be placed on the market or put into service after the IVDR’s application date, provided those devices remained compliant with the IVDD and had no significant changes in design or intended purpose. The IVDR obligations concerning post-market surveillance, vigilance, and the registration of economic operators and devices take precedence over and replace the equivalent stipulations in the IVDD.
On July 9, 2024, Regulation (EU) 2024/1860 was formally published, officially extending the transition periods for in vitro diagnostic medical devices. Regulation (EU) 2024/1860 not only addresses device transition but also incorporates a gradual implementation of the EUDAMED database, information requirements for supply interruptions, and revised transitional provisions for IVD medical devices.
Certificates that expired before the amending Regulation 2024/1860 are considered valid only if, prior to their expiration, the manufacturer and a Notified Body had a written agreement for conformity assessment of the device or its intended replacement, or if a national Competent Authority granted a derogation under Article 54(1) of the IVDR or mandated the manufacturer to complete the conformity assessment within a specified timeframe as per Article 92(1) of the IVDR. Even if a national derogation is time-limited or a conformity assessment deadline is imposed, the device still benefits from the complete transitional period until December 31, 2027, provided the conditions outlined in Article 110(3c) of the IVDR are met, with the certificate remaining valid until that date unless it is withdrawn.
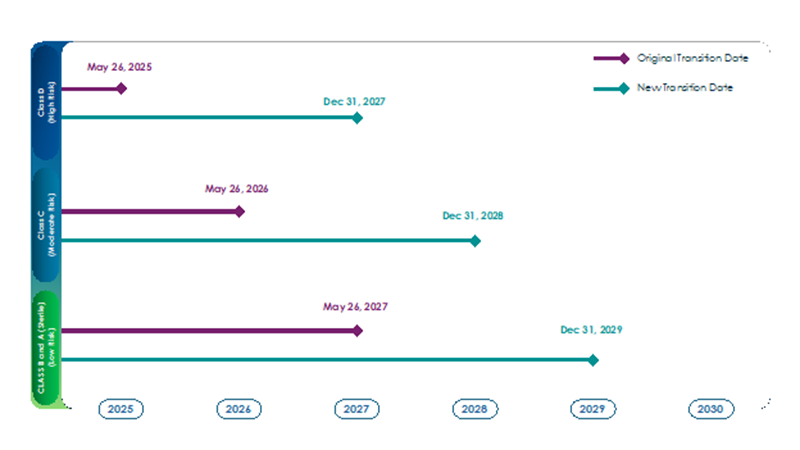
Under the new provisions, the additional time granted to manufacturers of IVDs depends on the type of device:
There is no transitional period for Class A devices (except sterile devices) because these devices do not require Notified Body involvement for their assessment. Similarly, “new” devices (those without an IVDD certificate or declaration of conformity) are also not covered in the transitional period. The IVDR has been in effect for these devices since May 26, 2022.
IVDR Transition Timeline
IVD Class | Risk Level | Original IVDR Transition Date | New IVDR Transition Date |
Class D | High | May 26, 2025 | Dec 31, 2027 |
Class C | Moderate | May 26, 2026 | Dec 31, 2028 |
Class B and Class A (sterile) | Lower | May 26, 2027 | Dec 31, 2029 |
Impact on Manufacturers and Economic Operators
The IVDR significantly impacts manufacturers by introducing a more stringent risk-based classification system, requiring increased technical documentation and mandating more rigorous post-market surveillance. These changes demand adjustments in product development, regulatory affairs, and the quality management system (QMS) for manufacturers seeking to keep and/or place their IVDs on the European market.
Actionable Steps for Compliance
It is critical to have a transition plan with a strategic alignment of development, testing, and regulatory approaches to the IVDR phased deadlines. Furthermore, early engagement with Notified Bodies is strongly advised to avoid potential certification delays due to the increased demand for their services.
The transition to the IVDR also entails increased investment in compliance, including clinical studies, post-market surveillance, and updated technical and QMS documentation, consequently increasing costs and resource allocation.
Economic operators (importers, distributors, and authorized representatives) must adhere to their corresponding responsibilities by the specified deadlines. Failure to comply with the requirements of the IVDR will lead to the removal of noncompliant devices from the market, potentially impacting revenue streams and patient access to critical diagnostics.
The IVDR transition is well underway, and manufacturers must stay ahead of compliance deadlines to ensure uninterrupted market access. Early planning, regulatory expertise, and strategic alignment with IVDR milestones are essential for success.
Sources:

US OfficeWashington DC
EU OfficeCork, Ireland




UNITED STATES
1055 Thomas Jefferson St. NW
Suite 304
Washington, DC 20007
Phone: 1.800.472.6477
EUROPE
4 Emmet House, Barrack Square
Ballincollig
Cork, Ireland
Phone: +353 21 212 8530