
Clients often ask us what has changed with regard to vigilance reporting in the European Medical Device Regulation (2017/745) and In Vitro Diagnostic Regulation (2017/746). Basically, not much, but there are a few important changes you should be aware of.
First, it is important to note that in October 2019, the European Commission published Additional Guidance Regarding the Vigilance System as outlined in MEDDEV 2.12-1 rev. 8. This additional guidance should be used in conjunction with MEDDEV 2.12-1 rev.8. Changes include:
Important: The EU MDR postmarket surveillance (PMS) requirements have been applicable since May 26, 2021 for all medical devices sold in the EU regardless of a device’s MDR CE Marking status. Medical device manufacturers need to ensure that their procedures are updated and employees responsible for vigilance reporting are trained on the change in reporting timeline.
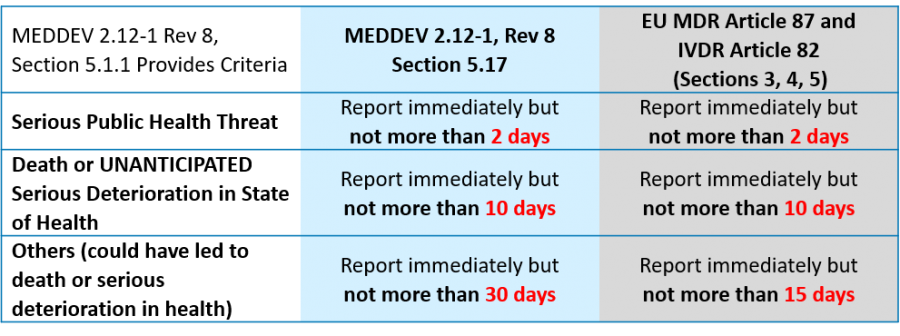
MEDDEV 2.12-1 Rev 8 provides guidance to medical device manufacturers on market surveillance. It continues to be the primary guidance document for vigilance reporting, even with the implementation of the new EU MDR and IVDR. However, Article 87 of the new EU MDR and Article 82 of the EU IVDR have shortened the timeline for vigilance reporting of serious incidents that did not lead to death or serious deterioration in health from 30 to 15 days. Because these regulations take precedence, it is anticipated that the guidance will be updated to be consistent with the new vigilance reporting requirements.
Any serious incident that occurs with a device that is not in the EU market and did not lead to a Field Safety Corrective Action (FSCA) is still not reported through vigilance in the EU.
Section 5.1, paragraph 4 of MEDDEV 2.12-1 Rev 8 was written to support the MDD, AIMDD, and IVDD vigilance reporting requirements, and very clearly states the following:
“INCIDENTs which occurred outside the EEA, Switzerland and Turkey do not lead to a FIELD SAFETY CORRECTIVE ACTION relevant to these geographic areas do not need to be reported.” “Outside the EEA, Switzerland and Turkey” means any country that has not placed the device on the market under one of the directives, or more specifically (in the current regulation language) within the “Union Market.”
In case you are wondering, the UK is still operating under the EU device directives in that general medical devices, including custom-made devices, compliant with the EU medical devices regulation (EU MDR) and IVDs compliant with the EU in vitro diagnostic medical devices regulation (EU IVDR) can be placed on the Great Britain (UK) market up until the 30 June 2030.
Subsequent to that guidance, the EU MDR and IVDR were released, and there has been widespread confusion around the new language in paragraph 1a of Article 87 of the EU MDR (Article 82 in IVDR) for reporting of serious incidents, which states:
“… any serious incident involving devices made available on the Union market…”
It could be interpreted (that would mean) that if a certain model of device has been distributed in the EU market and an event occurs outside of the EU market, then since that same model of device is “available on the EU market” it would now need to be reported.
However, look at the wording in paragraph 1b of MDR Article 87 (Article 82 in IVDR) for reporting, which states:
“…any field safety corrective action in respect of devices made available on the Union market, including any field safety corrective action undertaken in a third country in relation to a device which is also legally made available on the Union market, if the reason for the field safety corrective action is not limited to the device made available in the third country.”
It becomes clear from the statement in paragraph 1b that the same reporting requirements remain in effect as per the MEDDEV 2.12.1 guidance. Specifically, that reporting is only required for serious incidents occurring for events where the device is actually in the EU market, unless an event occurring outside the EU leads to an FSCA affecting devices in the EU.
If you still think the new language in paragraph 1a requires you to report vigilance for serious incidents that occur with devices outside of the EU market, to which Competent Authority would you report the incident? The reporting of incidents under the new regulations is based on future use of an electronic system (EUDAMED). Note that Article 92, Section 5 of the MDR states:
“The reports on serious incidents referred to in point (a) of Article 87(1) shall be automatically transmitted, upon receipt, via the electronic system referred to in paragraph 1 of this Article, to the competent authority of the Member State in which the incident occurred.”
There clearly is no relevant “Member State” competent authority for serious incidents that occur outside of the EU market.
With the EU MDR implementation fast approaching, you need to stay updated on its impact on your company. Consider our EU MDR auditing class, our IVDR overview class, or this focused training course on medical device complaint management and vigilance.

US OfficeWashington DC
EU OfficeCork, Ireland



UNITED STATES
1055 Thomas Jefferson St. NW
Suite 304
Washington, DC 20007
Phone: 1.800.472.6477
EUROPE
4 Emmet House, Barrack Square
Ballincollig
Cork, Ireland
Phone: +353 21 212 8530